The Backboard
VOCABULARY
Annealed: A procedure of slowly cooling glass to relieve internal stresses and increase the strength of the glass.
Brittle: Easily broken or shattered; not flexible or resilient.
Cobalt: A chemical element (Co) that is a silvery-gray, magnetic metal often used in alloys and magnets.
Coil spring: A type of spring made of wire wound into a spiral shape that provides elasticity.
Constituent: A part or component of a whole.
Deformation: A change in shape or form, often caused by external forces.
Equivalent: Having the same value, function, or meaning as something else.
Ferri-magnetic: Having magnetic properties, capable of being attracted to a magnet.
Ferromagnetism: The phenomenon where certain materials become strongly magnetized in the presence of a magnetic field.
Granular: Composed of small, grain-like particles.
Mediated: Transmitted or conveyed through a medium or agent.
Melting point: The temperature at which a solid changes into a liquid.
Obstruct: To block or hinder the view or passage of something.
Opaque: Not transparent or translucent; not allowing light to pass through.
Patent: A government grant that gives exclusive rights to an inventor to produce and sell an invention.
Pivot: A point or axis on which something turns or rotates.
Prisms: Transparent objects with flat surfaces that can refract light and split it into its component colors.
Refract: To bend or change the direction of light as it passes through a medium, such as glass.
Shards: Small, sharp fragments or pieces, especially of glass.
Silica: A chemical compound, silicon dioxide (SiO2), which is the main component of sand and quartz.
Silicate: A chemical compound containing silicon and oxygen, such as quartz, feldspar, and mica.
Sodium oxide: A chemical compound, Na2O, commonly known as soda, used in glassmaking.
Splintering: Breaking or splitting into sharp, thin pieces.
The Backboard
A backboard is a raised vertical board with a basket attached. It is made of a flat, rigid piece of material, usually rectangular as used in NBA, NCAA and international basketball. Today most professional backboards are made of a glass backboard so that it will not obstruct the audience's view.

Regulation backboards are 6 feet wide (72 inches) by 42 inches tall. All basketball rims (hoops) are 18 inches in diameter. The inner square on the backboard is 24 inches wide by 18 inches tall.
The first glass backboard was used at the Men's Gymnasium at Indiana University. In 1917, spectators complained that they couldn't see the game because of opaque wooden backboards. As a result the new backboards that contained 1 ½” thick plate glass so that fans could see games without an obstructed view.

Glass
Glass is a non-crystalline (a crystal is a solid material whose arranged in a highly ordered structure), amorphous (lacking a clear structure), solid that is often transparent and has widespread practical, technological, and decorative usage in things like window panes, drinking glasses and eyeglasses.
In nature, glass formation of quartz (SiO2, also known as Silicon dioxide) occurs when lightning strikes sand. Fused quartz is a glass made from chemically-pure silica. However, its high melting-temperature makes it difficult to work with. In 1945, the first-ever nuclear bomb blast produced such extreme heat that it melted the surrounding sandy soil into a green, radioactive glass for about 1,150 feet in all directions from ground zero.
The most familiar, and historically the oldest, types of glass are based on the chemical compound silica (silicon dioxide), the primary constituent of sand. The term glass, in popular usage, is often used to refer only to this type of material, which is familiar from use as window glass and in glass bottles. Of the many silica-based glasses that exist, ordinary glass is formed from a specific type called soda-lime glass, composed of approximately 75% silicon dioxide (SiO2), with the remainder consisting of sodium oxide (Na2O) from sodium carbonate (Na2CO3), calcium oxide(CaO), known popularly as lime and several minor additives. In glasses like soda lime, the other compounds are used to lower the melting temperature and improve the temperature workability of the product at a cost in the toughness, stability, and optics.
Glass will transmit, reflect and refract light; these qualities can be enhanced by cutting and polishing to make optical lenses, prisms, fine glassware, and optical fibers for high speed data transmission by light. Glass can be colored by adding metallic salts, and can also be painted. These qualities have led to the extensive use of glass in the manufacture of art objects and in particular, stained glass windows. Although brittle, silicate glass is extremely durable, and many examples of glass fragments exist from early glass-making cultures. Because glass can be formed or molded into any shape, and also because it is a sterile product, it has been traditionally used for vessels: bowls, vases, bottles, jars and drinking glasses.
Tempered Glass
Toughened or tempered glass is a type of safety glass processed by controlled thermal or chemical treatments to increase its strength compared with normal glass. Tempering puts the outer surfaces into compression and the interior into tension. Such stresses cause the glass, when broken, to crumble into small granular chunks instead of splintering into jagged shards as plate glass does. The granular chunks are less likely to cause injury.
As a result of its safety and strength, tempered glass is used in a variety of demanding applications, including passenger vehicle windows, shower doors, architectural glass doors and tables, refrigerator trays, mobile screen protectors, as a component of bulletproof glass, for diving masks, and various types of plates and cookware.
Toughened glass is physically and thermally stronger than normal glass. The greater contraction of the inner layer during manufacturing induces compressive stresses in the surface of the glass balanced by tensile stresses in the body of the glass. As a result of the increased surface stress, if the glass is broken it only breaks into small circular pieces as opposed to sharp jagged shards.
Tempered glass is used when strength, thermal resistance, and safety are important considerations. Passenger vehicles, for example, have all three requirements. Since they are stored outdoors, they are subject to constant heating and cooling as well as dramatic temperature changes throughout the year. Moreover, they must withstand small impacts such as from road debris such as stones as well as automobile accidents. Because large, sharp glass shards would present additional and unacceptable danger to passengers, tempered glass is used so that if broken, the pieces are blunt and mostly harmless.
The windscreen or windshield is instead made of laminated glass (a type of safety glass that holds together when shattered. In the event of breaking, it is held in place by an interlayer, of vinyl, a form of plastic, between its two or more layers of glass. The interlayer keeps the layers of glass bonded even when broken, and its high strength prevents the glass from breaking up into large sharp pieces. This produces a characteristic "spider web" cracking pattern when the impact is not enough to completely pierce the glass. The side windows and the rear windshield is typically tempered glass.
Other typical applications of tempered glass include:
• Balcony doors
• Athletic facilities (backboards)
• Swimming pools
• Building facades
• Shower doors and bathroom areas
• Exhibition areas and displays
Tempered and heat strengthened glass can be three to seven times stronger than annealed (typical) glass. Building codes in the United States require tempered or laminated glass in several situations including some skylights, near doorways and stairways, large windows, windows which extend close to floor level, sliding doors, elevators, fire department access panels, and near swimming pools.
Manufacturing
Tempered glass can be made from annealed glass via a thermal process. The glass is placed onto a roller table, taking it through a furnace that heats it well above its transition temperature of 564 °C (1,047 °F) to around 620 °C (1,148 °F). The glass is then rapidly cooled with forced air drafts while the inner portion remains free to flow for a short time.
An alternative chemical toughening process involves forcing a surface layer of glass at least 0.1 mm thick into compression by ion exchange of the sodium ions in the glass surface with potassium ions (which are 30% larger), by immersion of the glass into a bath of molten potassium nitrate. Chemical toughening results in increased toughness compared with thermal toughening and can be applied to glass objects of complex shapes.
Disadvantages
Toughened glass must be cut to size or pressed to shape before toughening, and cannot be re-worked once toughened. Polishing the edges or drilling holes in the glass is carried out before the toughening process starts. Because of the balanced stresses in the glass, damage to any portion will eventually result in the glass shattering into thumbnail-sized pieces. The glass is most susceptible to breakage due to damage to the edge of the glass, where the tensile stress is the greatest, but shattering can also occur in the event of a hard impact in the middle of the glass pane or if the impact is concentrated.
Using tempered glass can pose a security risk in some situations because of the tendency of the glass to shatter completely upon hard impact rather than leaving shards in the window frame.
Backboard (Glass) Shattering
Backboard shattering is an accident or stunt in basketball. It occurs when a player slam dunks the ball hard enough to break the 1.5” glass of the backboard.
In 1967, the dunk was banned in high school and college basketball. The rule-makers claimed the dunk was outlawed to prevent injury and equipment damage.
The first NBA player to shatter a backboard, Chuck Connors (who would become far more famous as an actor), did not do so with a dunk. When playing for the Boston Celtics in 1946, Connors took a set shot during pregame warmups, hitting the front of the rim. Because an arena worker had failed to place a protective piece between the rim and backboard, the backboard shattered.
During the 1970s, NBA players like Julius Erving (Dr. J), popularized the dunk with their athletic flights to the basket. Dunking acquired a “macho mystique” and this was taken to an extreme by Darryl Dawkins.

In 1976 the NCAA allowed the slam dunk to be legal again due to the invention of the breakaway rim.
The Breakaway Rim
The top of the basketball hoop is 10 feet above the ground and all basketball rims (hoops) are 18 inches in diameter. It is mounted to a basketball backboard via a flexible connection between the backboard and the connection supporting the hoop. In a breakaway rim, the shock of a basket or a dunk is absorbed by the connecting part, so that the rim goes back to a horizontal position.

A breakaway rim can bend slightly downward when a player dunks a basketball, and then instantly snap back into a horizontal position when the player releases it. Pivotal movement is provided by a hinge or a ball-and-socket connector. A coil spring behind the goal backboard and connected to the bracket by a spring to automatically return the temporarily displaced goal to its normal position. It allows players to dunk the ball without shattering the backboard.
Arthur Ehrat, the inventor of the breakaway rim, worked at a grain elevator for most of his life and barely knew anything about basketball. In 1975, his nephew, an assistant basketball coach at Saint Louis University, asked him to help design a rim that could support slam dunks. Using a spring from a piece of farm machinery, he designed a rim that could bend and spring back after 125 pounds of force were applied to it. He called his device "The Rebounder". In 1982, he was awarded a patent for his invention, which was officially called a "deformation-preventing swingable mount for basketball goals".

In Ehrat’s description of his device he mentioned that…strong magnets (since replaced by coilsprings) or their equivalent structure firmly hold the bracket against movement by normal game-applied forces, such as non-dunk shots. The word “magnet” usually refers to one of the hoop’s most common nicknames, the iron. This nickname is well deserved because the hoop is primarily composed of the element iron (Fe) in its most common form magnitite (Fe3O4).
The Chemical Composition of a Basketball Hoop
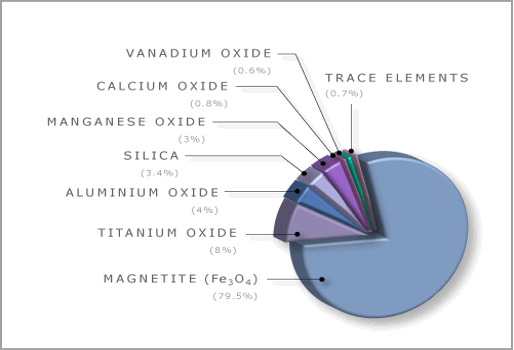
Magnetite
Magnetite is ferri-magnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. It is the most magnetic of all the naturally-occurring minerals on Earth. Naturally-magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism. Ferromagnetism is responsible for most of the effects of magnetism encountered in everyday life.
Magnetism is a class of physical phenomena that are mediated by magnetic fields. Electric currents and give rise to a magnetic field, which acts on other currents. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetized to become permanent magnets, producing magnetic fields themselves. Only a few substances are ferromagnetic; the most common ones are iron, nickel and cobalt.
STEM Topic 06: Problem Set
-
Iron has an atomic number of 26. How many protons does an iron atom contain?
-
The molecular formula of nylon-6,6 is (C12H22N2O2). How many carbon atoms are present in one molecule of nylon-6,6?
-
The density of iron is 7.87 grams per cubic centimeter. What is the mass of a 5 cm x 2 cm x 2 cm block of iron?
-
Nylon-6 is formed by the polymerization of a molecule caprolactam (C6H11NO). How many nitrogen atoms are present in one molecule of caprolactam?
-
The molar mass of iron is 55.85 grams per mole. What is the mass of 0.5 moles of iron?
-
Nylon is a thermoplastic polymer. If a nylon thread has a melting point of 220 degrees Celsius and is subjected to a temperature of 180 degrees Celsius, is it likely to melt?
-
A square iron plate has a side length of 12 centimeters. What is its perimeter?
-
A circular glass tabletop has a diameter of 80 centimeters. What is its circumference?
-
A triangular piece of nylon fabric has a base of 10 meters and a height of 6 meters. What is its area?
-
A cylindrical iron rod has a radius of 4 centimeters and a height of 10 centimeters. What is its volume?
-
A regular hexagonal piece of nylon fabric has a side length of 7 meters. What is its perimeter?
-
A cone-shaped iron bell has a radius of 6 centimeters and a height of 8 centimeters. What is its volume?
-
A cylindrical glass vase has a radius of 5 inches and a height of 12 inches and a flat top piece. What is its exterior surface area?
-
A parallelogram-shaped piece of nylon fabric has a base of 15 meters and a height of 9 meters. What is its area?
![student scientist6c[1].png](https://static.wixstatic.com/media/cfa49f_4db66c81df244b1e9d77c3f269ddb33d~mv2.png/v1/fill/w_73,h_94,al_c,q_85,usm_0.66_1.00_0.01,enc_auto/student%20scientist6c%5B1%5D.png)